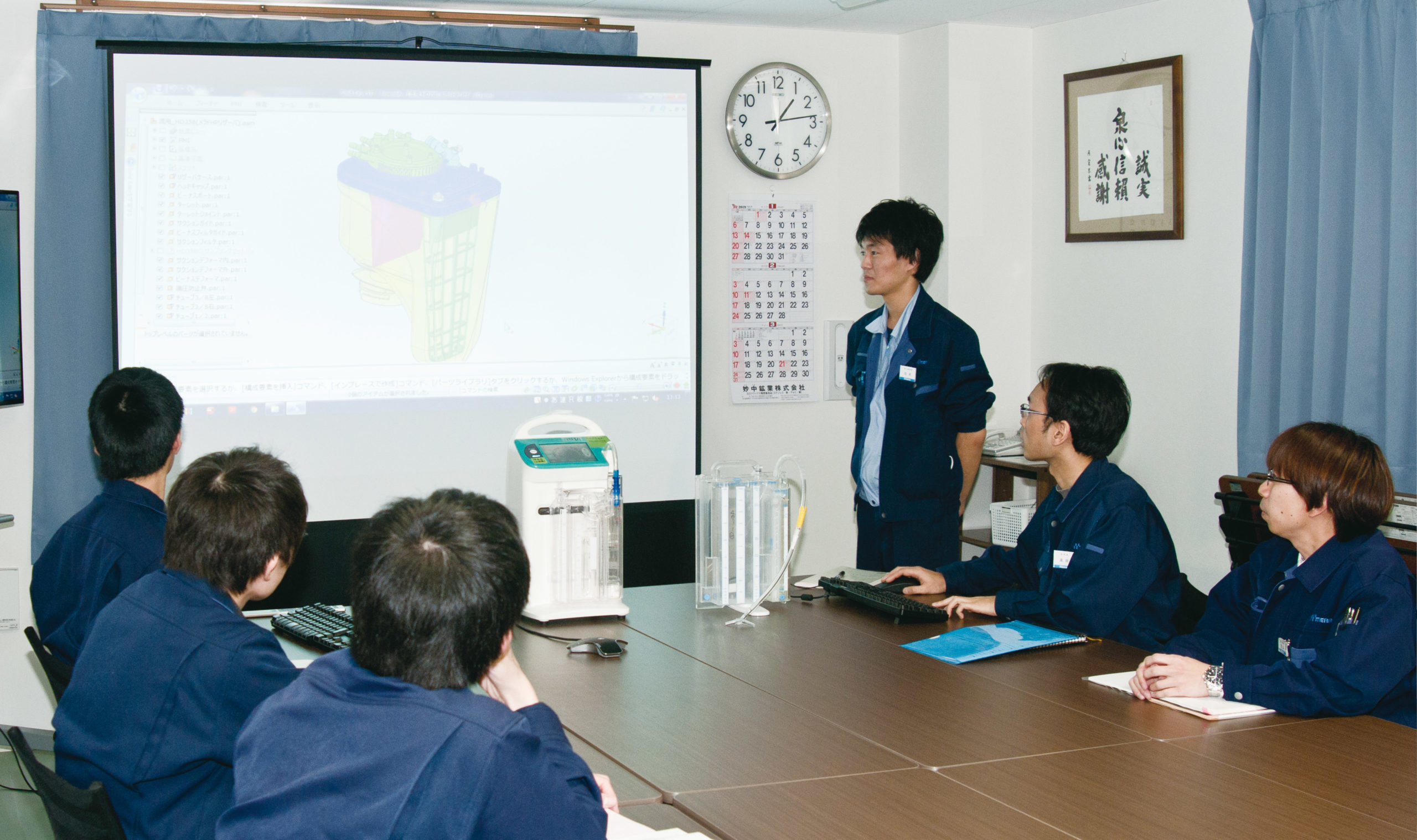
High reliability is required for medical devices related to the maintenance of life. In development meetings held repeatedly, therefore, the members review and discuss structure/function, manufacturing method and maintenance of the medical devices over and over again. We try to develop and improve the required technologies to find higher reliability. In addition, we assume possible problems with products during use as much as possible. Finally, analyzing risk management from the initial stage of the development of a product, our team tries to take the safety of a product into careful consideration.

Various verification tests are carried out repeatedly regardless of whether an improved article or a new product in the trial manufacture stage. We will confirm the validity through testing from many aspects: does the device show adequate performance and function? Does the device have any safety or durability problems? Does the device have efficient original functions in any situation? Is there any influence on the patient’s other organs? These results and test data used in producing products with higher quality.

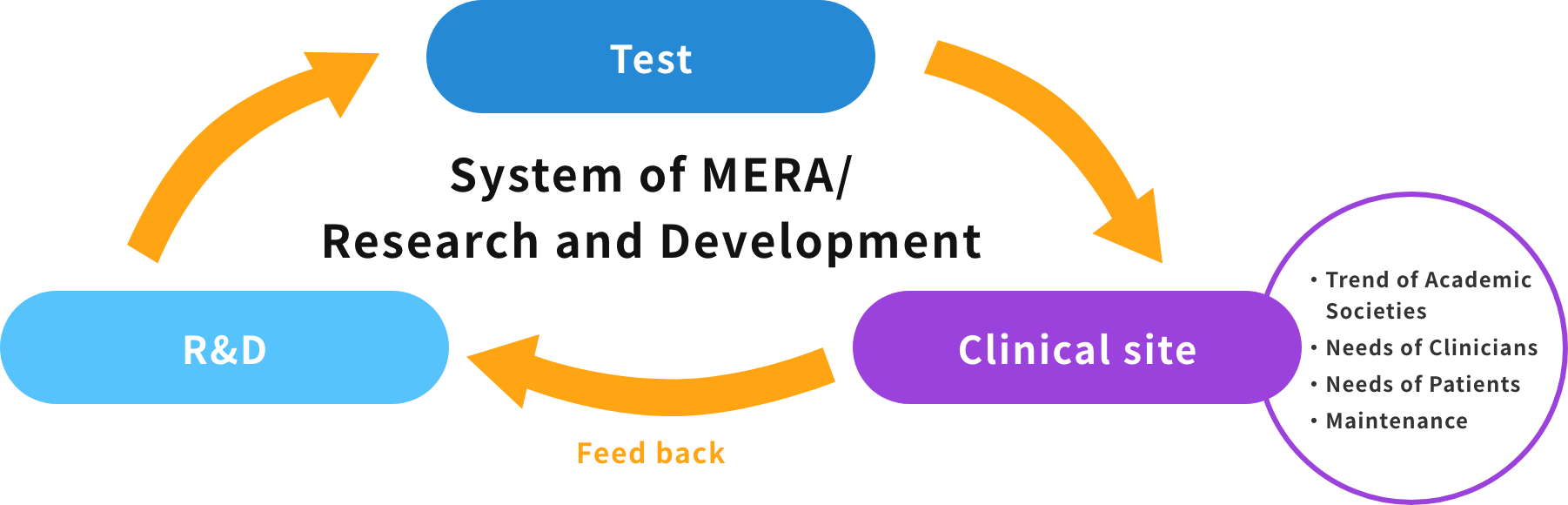
With the advance of clinical medicine, active technical innovation is necessary for the consistent research and development of medical devices. This should provide better reliability that is brought about by cooperative activities between clinical study sites and medical device manufacturers. While deeply understanding the situation described above, we actively address the development of therapeutic medical devices directly related to clinical sites, based on our abundant experience and performance, including past results such as the development of the first heart-lung machine in Japan.

As our company produces devices and equipment used for treatment in medical care facilities, we are wholly committed to providing the utmost assurance of their effectiveness and safety. The ISO 13485 certification standard established a product quality management system that ensures fulfillment of universal design & production management standards and encourages maintenance of quality and continuous improvement. The ISO 13485 standard added special requirements unique to medical equipment, such as sterilization validation, to the general product quality management system established by the ISO 9001 standard. Our company has been certificated as conforming to this standard by the BSI Group Japan.


As science and medicine continue to advance, so must the quality of our products. However, as medical equipment directly impacts the health and well-being of patients, our company provides quality assurance not only in our manufacturing process but has created, has implemented, maintains, and continuously improves a management system based on the PDCA Plan→Do→Check→Act cycle that is applied across the board, from the provision of customer information to purchasing to planning & development, storage, delivery, and maintenance services. Furthermore, we maintain a product quality management system that assures quality by preserving traceability through our data management system, as well as striving to improve product quality continuously through risk management. This system fosters improvement via daily reviews to strengthen the bonds of trust between our company and our client doctors and patients.